|
| Adrenal Function Urine Test |
| Sulkowitch Urine (Calcium) Test |
|
|
|
TIN
Number 50 on the "periodic table" of elements
Tests the presence of ions of tin to a high degree of accuracy to
detect contamination in the body and on a wide range of materials in your environment
Tin is one of the earliest metals known and used. About 35 countries mine tin throughout the world. Nearly every continent
has an important tin-mining country. Tin is a relatively scarce element with an abundance in the earth's crust of about
2 parts per million (ppm), compared with 94 ppm for zinc, 63 ppm for copper, and 12 ppm for lead. Most of the world's tin
is produced from placer deposits; at least one-half comes from Southeast Asia. The only mineral of commercial importance as
a source of tin is cassiterite (SnO2), although small quantities of tin are recovered from complex sulfides such as stanite,
cylindrite, frankeite, canfieldite, and teallite.
Most tin is used as a protective coating or as an alloy with other metals such as lead or zinc. Tin is used in coatings for
steel containers, in solders for joining pipes or electrical/electronic circuits, in bearing alloys, in glass-making, and in
a wide range of tin chemical applications. Secondary, or scrap, tin is an important source of the tin supply.
Tin resists distilled, sea, and soft tap water, but is attacked by strong acids, alkalis, and acid salts. Oxygen in solution
accelerates the attack. When heated in air, tin forms SnO2M, which is a feeble acid, forming stannate salts with
basic oxies. The most important salt is the chloride which is used as a reducing agent and as a mordant in calico printing.
It is, or was, used to plate steel, making "tin cans".
Sources
Tin is found chiefly in cassiterite. Tin is obtained by reducing the ore with coal in a reverberatory furnace. The small amount of tin found
in canned foods is quite harmless.
Toxicity and Symptoms
Triethyltin is the most dangerous organic tin substance for humans. Humans can absorb tin bonds through food and breathing and through the
skin.
Toxicity Limits
The estimated daily tin intake in food and water is (including canned food) is 1 -3 ppm daily There is cause for concern when the daily
intake exceeds 200 ppm which is the maximum legal limit allowed in canned foods. Ideally concentration levels should be 0.
Check out tin levels in your body with our easy to use, home-based,
HMT Tin Test kit
 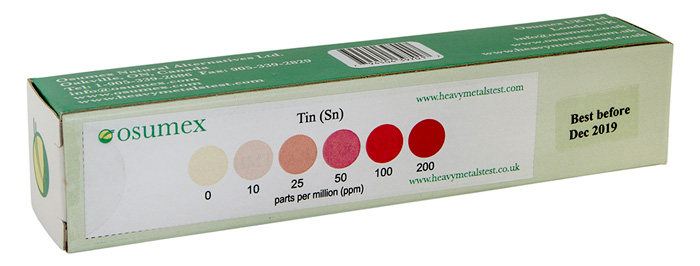
Sample of a HMT Tin Test kit
Osumex HM-Chelat is most effective in eliminating heavy metals contamination in the
body

The above information is provided for general
educational purposes only. It is not intended to replace competent
health care advice received from a knowledgeable healthcare professional.
You are urged to seek healthcare advice for the treatment of any
illness or disease.
Health Canada and the FDA (USA) have not evaluated these
statements. This product is not intended to diagnose, treat, cure, or prevent
any disease.
|


